LEoPARD has presented its research in poster format at a national meeting, the 2024 Canadian Society for Chemistry annual meeting. This is an updated and revamped version of the work that was presented at the institutional meeting, the 2023 Saint Michael’s College Academic Symposium.
This is an exciting accomplishment, marking the first national meeting at which we’ve been fortunate to present our research, since becoming an independent research group in August 2019!
The poster was presented in the Biological and Medicinal Chemistry Division, as part of the Monday evening poster session. Our abstract, as recorded for the meeting, is as follows:
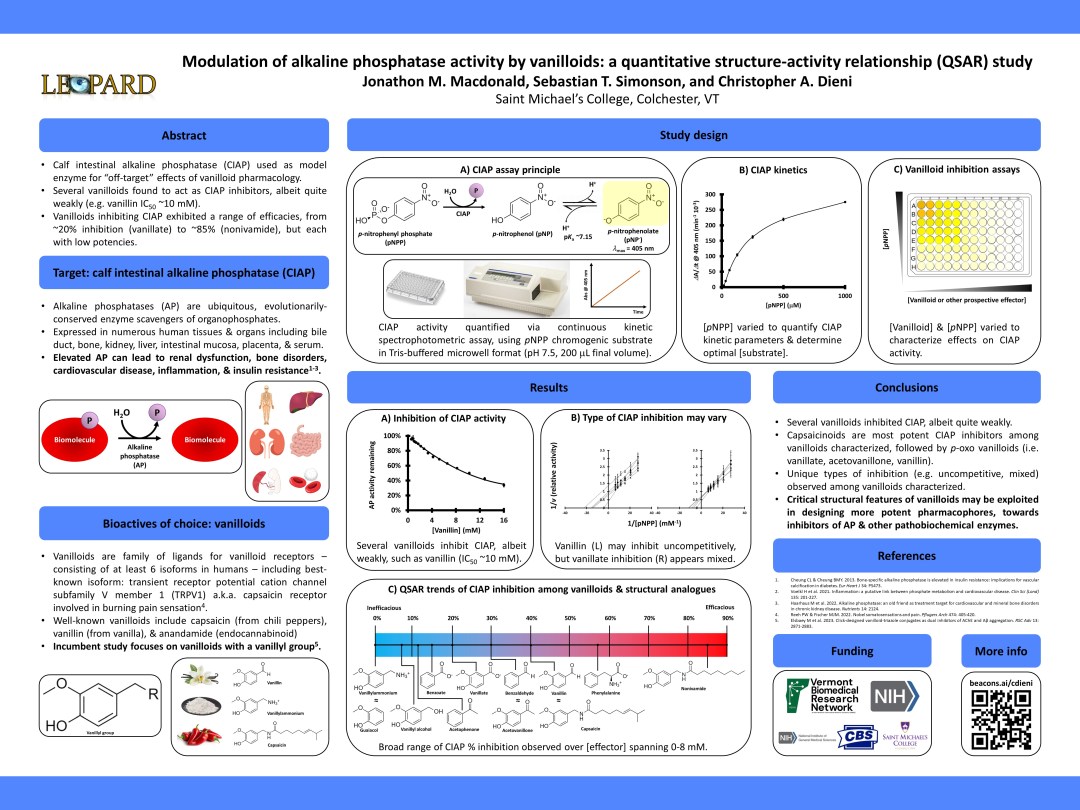
Vanilloids are a class of naturally-occurring compounds, with standout examples including vanillin (the fragrant compound in vanilla) and capsaicin (the spicy compound in chili peppers). Past studies in animal and cellular models have suggested that some vanilloids have purported antidiabetic bioactivities; however, their mechanisms of antidiabetic action have yet to be unequivocally elucidated. While the putative receptors for vanilloids are those of the transmembrane transient receptor potential cation channel subfamily V (TrpV), in this study we have used calf intestinal alkaline phosphatase (CIAP) as a simple protein model for preliminary studies of “off-target” or additional vanilloid pharmacodynamics and bioactivities. Alkaline phosphatase (AP) is a ubiquitous enzyme found in numerous cell and tissue types in addition to the intestine, and catalyzes hydrolysis of phosphoryl moieties from organophosphates (R-O-PO32-) for a variety of metabolic purposes. However, dysfunction of AP leads to a plethora of human diseases; for instance, elevated levels of serum AP have been linked to a series of metabolic disorders including insulin resistance, which can lead to development of type 2 diabetes. Here, we present the results of kinetic enzyme assays using commercially-available calf intestinal alkaline phosphatase (CIAP) and varying concentrations of vanilloids, to establish the quantitative structure-activity relationships by which vanilloids may modulate CIAP. We have found correlations between structural features and functional groups within the vanilloids and resulting inhibition of CIAP. These results are quantitatively and qualitatively defined herein.